Georgia Podiatrist Turns to Memphis Experts to Bring Innovative Surgical Device to Market: ARROW-LOK[TM] Digital Fusion System Offers an Alternative for “Hammertoe” Patients
Memphis, TN
September 24, 2012
As a podiatrist Scott Roman, DPM, has helped countless patients suffering from proximal interphalangeal joint flexion deformity, commonly known as “hammertoes.” This is a progressive and often painful condition in which the toes are held in a flexed or bent position. Now — thanks to the innovative ARROW-LOK™ Digital Fusion System that Roman invented — patients across the country who undergo hammertoe surgery can enjoy a more comfortable post-operative experience.
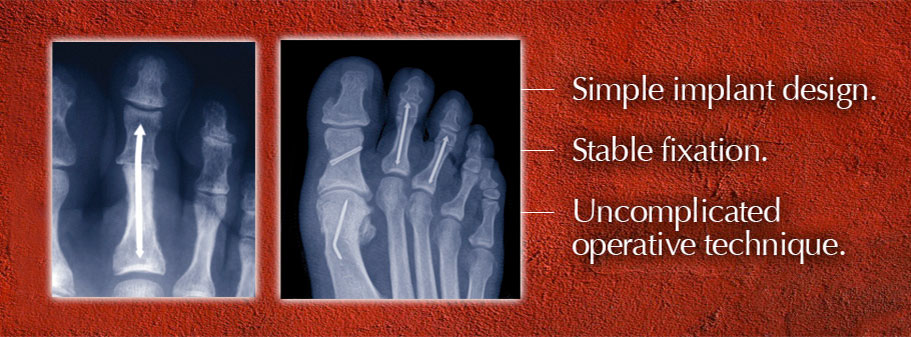
The traditional surgical approach for correcting hammertoes utilizes a Kirschner wire or “K-wire” to hold the bones of the toes in position after surgery so they can fuse into the correct alignment. The wire is usually left protruding from the toe for four to six weeks after surgery. “Not surprisingly,” Roman notes, “that causes anxiety for many patients, who worry about stubbing the toe with the wire sticking out, or even accidentally pulling it out.”
There are other difficulties related to use of external K-wires: They are left sticking out of the body, which can increase the risk of postoperative infection; the wires have to be removed before the bone has completely healed, which can be an uncomfortable procedure for the patient; and K-wires may simply not be stable enough, leading to internal instability or even failure of the fusion. “I used to embed a short wire to provide a balance of effectiveness and comfort for my patients,” Roman says, “but stability was not as solid as I wanted it to be. The segments of the toes would sometimes distract off the wire or gap at the fusion site.”
“K-wires have been in use for over 40 years,” Roman says. “We needed to overcome the challenges of the current procedure and create a better approach.”
Roman envisioned an arrow-shaped device that could be used in place of the traditional external wire. The challenges of taking a medical device through the FDA clearance process and creating a national distribution channel, however, presented challenges that Roman, already involved in a busy medical practice, could not meet on his own. “I had ideas, I had prototyping,” Roman says, “but I didn’t have a team of experienced professionals supporting me.”
At the urging of a professional associate, Roman connected with Patrick Mullaney, whose 20-year career has been largely focused on selling and commercializing medical devices. Recognizing the originality and market potential of Roman’s hammertoe device, Mullaney and Tom Twardzik, a professional associate with 25 years in the industry, co-founded Arrowhead Medical Device Technologies, LLC, in 2010. The company is focused on introducing new devices for treatment of musculoskeletal disorders.
Roman’s hammertoe solution became the company’s first product. The initial design received 510(k) FDA clearance in the fall of 2010, which meant that doctors could start using the product in patients. Feedback from surgeons led to development of a “second generation” implant design that made the product easier to use and able to fit a wider range of patient anatomies. Cleared by the FDA in October 2011, the ARROW-LOK Digital Fusion System has since been launched in over 20 of the nation’s key markets.
Launching a new medical device is “a daunting task,” Mullaney says. “You have to finalize the design, implement production, develop a compelling message, and create a distribution network. Your people on the street personally present the product to the surgeons and support its use in the OR. Sales reps are the actual human contact you have with the users, with hospitals and surgery centers. We are very selective about who represents ARROW-LOK technology, and work through a network of independent agents in strong markets. That’s allowing us to rapidly gain acceptance of the ARROW-LOK system among leading podiatric and orthopaedic surgeons.”
Since the system’s availability in December 2010, Roman has used the system on his own patients, “both young and old, with excellent results. The system is designed to maintain stability until the bone is fully healed, to reduce patient anxiety about the removal of external wires and to reduce the possibility of postoperative infections.”
Roman says, “I’ve had several patients that have been putting off surgery for years because they didn’t want to go through the experience with the external wires, the most common manner of fixation. The ARROW-LOK system is a comparatively anxiety-free post-op experience for them. As we bring the device to doctors across the country, the patient population will become more aware of the product, and discover that they can have hammertoe surgery without wires protruding from the ends of their toes. That’s when they will begin requesting to be treated with a product like the ARROW-LOK device.”
In determining whether surgery is appropriate for a given patient, Roman always starts with the most basic, and important, consideration: “The first question I ask patients is if they are experiencing pain that alters their activities of daily living. When the benefits outweigh the risks of surgery, that’s the time to have the procedure to get the toes corrected.”
Roman is so confident of the ARROW-LOK design that he recommended it for his own mother, who had experienced pain and physical difficulties after two surgeries using K-wires had failed. “One was an internal wire and one was external, but they both failed,” Roman says. “The ARROW-LOK device worked well on my mom, and now she’s fine. It’s gratifying to watch her keep up with my kids without the discomfort that caused her to limp.”
“That’s really the point of creating this new system,” Roman continues. “I want to help my patients regain what they’ve lost. If you can wear shoes comfortably and do the things you want to do, it’s a great improvement in the quality of life.”
———————-
Founded in August 2010, by Patrick Mullaney, President, and Tom Twardzik, Vice President, Sales and Marketing, Arrowhead Medical Device Technologies, LLC, specializes in rapidly developing, manufacturing and distributing medical devices utilized in the treatment of musculoskeletal conditions. The headquarters is located just outside of Memphis, Tennessee, a region recognized as one of the world’s premier sites for musculoskeletal medical device innovation. Arrowhead received clearance to market its initial product, the ARROW-LOK™ Digital Fusion System in October 2010, performed the first surgeries in December 2010 and commenced unrestricted distribution in February 2011. Arrowhead plans to expand its product lines to include additional sizes and applications for the ARROW-LOK technology. In addition, the management of Arrowhead is actively seeking innovative medical device technologies that serve to improve patient care in an effective and affordable manner. (www.arrowheaddevices.com)
The Ankle and Foot Centers of Georgia is a long-established group of Board Certified podiatric physicians who are highly skilled innovators in the field of foot and ankle medicine. Founded in 1982 on the principles of technical excellence and personal concern for their patients’ health and well-being, the Centers’ doctors strive to provide patients with the latest available treatment options from conservative care to surgical intervention. (www.ankleandfootcenters.com)
———————-